Warning contains hard hitting photographs below
Chemical Injuries
Many chemicals present a serious hazard of causing a chemical injury
At home, products such as drain cleaners, oven cleaners, brick cleaners and rust removers, have the potential to cause a chemical injury if they come into contact with the skin or the eye.
At work, the risks increase significantly because of the volume, concentration and variety of chemicals increases.
Associated problems are:
- Being able to identify the chemical.
- The lack of knowledge regarding the chemical’s mechanism.
- The lack of synergy between the chemical and the emergency management protocol.
The consequences following a splash with an aggressive chemical can result in a chemical injury (lesion)
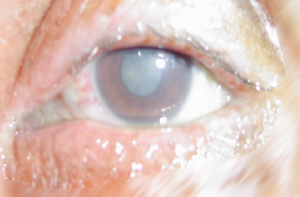
Hydrochloric Acid
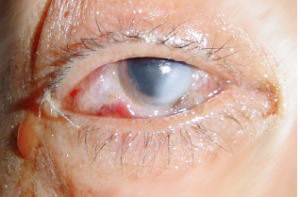
Sodium Hydroxide
What is a chemical injury (lesion)?
A chemical lesion is the local result of the reaction that corrosive or irritant chemical products have with biological tissue.
The chemical lesion is characterised by partial or total destruction of the biological tissue.
The severity of the lesion is proportional to the amount of tissue destruction: the severity of the chemical lesion is related to the type of chemical product, the nature of the tissue involved and the area affected.
The skin, eye, digestive and respiratory tract are in direct contact with the outside of the body and are therefore the primary targets of chemical products. Chemical lesions are injuries inflicted by contact between chemical products and these specific tissues.
The chemical lesion may also be accompanied by other systemic symptoms. When the chemical product causing the chemical lesion is toxic or harmful and passes into the body, reaching the organs or the bloodstream, it may interact with other biological targets.
Which chemical products cause a chemical Injury (lesion)?
The chemical products that may cause a chemical lesion are categorised according to the seriousness of the lesions that they may induce: they are either corrosive or irritant.
A chemical product is classified among corrosive or irritant agents when it is capable of reacting with biochemical components of the cells and the tissues of the skin, the eye and the respiratory or digestive tracts. The CLP/GHS regulations of the European Chemicals Agency provide the following definitions of corrosive or irritant products (CLP page L 353/87):
Skin corrosion means the production of irreversible damage to the skin, namely, visible necrosis through the epidermis and into the dermis, following the application of a test substance for up to 4 hours.
The reversibility of the skin lesions is to be considered in assessing the irritant nature of a substance.
A chemical is considered corrosive when it causes a severe lesion. Corrosive products cause destruction of the tissues with which they come into contact. A chemical will be considered irritant if it only causes irritation, redness or inflammation.
The same chemical product may be corrosive or only irritant depending on the circumstances:
- If present in a mixture or on its own
- Depending on its concentration
- Depending on the environment in which it is present
Some chemicals have the additional potential to cause other problems to human health such as those classified as toxic or harmful.
The hazard that a chemical product represents is indicated on its label by the manufacturer according to the regulatory obligations of the European Chemicals Agency.
Corrosive

Irritant
Chemical lesion or chemical burn?
Why talk about a chemical lesion rather than a burn?
Chemical lesion or chemical burn? Lesions on the human body caused by chemical products are very different from thermal burns. The mechanisms of development of these injuries are different: mere heat transfer is involved in the case of a thermal burn – a chemical reaction in the other case. The cicatrisation periods of both these types of injuries are variable. The semantic distinction is therefore also necessary.
What is the mechanism of a chemical lesion?
It all begins with a chemical reaction between two molecules. On a microscopic level, the chemical lesion results from a chemical reaction between an aggressive chemical product and a biological constituent.
This reaction destroys the biological constituent and hence the tissue, this forms the chemical lesion.
The mechanism involved in development of the lesion depends on many parameters, the most significant of which is the nature of the reaction initiated.
Depending on its type, each chemical product will react with a different component of the biological tissue by means of one of following actions:
- Acid-base reaction: H+ proton exchange reaction between an acid and an alkali.
- Redox reaction: electron exchange reaction between an oxidising agent and a reducing agent.
- Chelation: or formation of a complex from two molecules.
- Solvation: dissolution of a chemical species in a solvent.
Examples:
Acid-Base reaction between the hydroxide ions of caustic soda and the ester bonds of fats.
Solvation of the lipids of the cell membranes by hydrophobic solvents (diesel oil, toluene).
Chelation of the calcium ions contained in the cells by fluoride ions (originating for example from Hydrofluoric acid).
There are as many reactions that may cause chemical lesions as pairs of aggressive chemical product/biological target.
